
Cold Chain Logistics for GLP-1 Medications Explained

If you're using GLP-1 medications like semaglutide for weight loss or diabetes, keeping them at 2–8°C is critical. These peptide-based drugs are highly sensitive to heat and freezing, and even brief temperature changes can make them ineffective. Here's what you need to know:
- Why It Matters: GLP-1 medications, such as Ozempic and Wegovy, are biologics that degrade outside their safe temperature range, impacting their ability to regulate blood sugar and appetite.
- How It's Done: From manufacturing to delivery, cold chain logistics use insulated packaging, refrigerated transport, and temperature monitoring to maintain 2–8°C. This is especially challenging in Singapore's hot, humid climate.
- Your Role: When your medication arrives, check for temperature indicators, ensure the packaging is cool, and refrigerate it immediately. Store it in the main fridge compartment, away from the freezer or door.
Telehealth services in Singapore, like Trimly, follow strict regulations to ensure safe delivery of GLP-1 medications, using validated cold chain systems and real-time monitoring. Proper handling at every stage - from factory to your fridge - ensures your medication remains effective.
What Are GLP-1 Medications and Why Do They Need Cold Chain Management?
How GLP-1 Medications Work
GLP-1 medications are receptor agonists that imitate a natural hormone in the body responsible for regulating blood sugar levels and appetite. When these medications are administered, they bind to GLP-1 receptors found in the pancreas, gut, and brain. In the pancreas, they help boost insulin production when blood sugar rises after eating, while also reducing the release of glucagon - a hormone that prompts the liver to release stored glucose. Additionally, they slow down the emptying of the stomach, which helps smooth out blood sugar spikes. By targeting the brain's appetite centres, these drugs help reduce hunger and enhance feelings of fullness, promoting gradual weight loss when paired with a healthy diet and lifestyle modifications.
Some well-known brands include semaglutide-based options like Ozempic and Wegovy, as well as tirzepatide-based medications. In Singapore, these medications are commonly prescribed through specialist clinics, hospitals, and approved telehealth providers such as Trimly. Clinical studies highlight their effectiveness, showing that GLP-1 medications can lead to up to six times more weight loss compared to diet and exercise alone, with average weight reductions ranging from 5–15%. For example, the 68-week STEP 1 trial revealed that adults taking Wegovy (semaglutide 2.4 mg), alongside diet and exercise, experienced an average weight loss of around 15%, compared to just 2–3% with diet and exercise alone.
Given their precise biological mechanisms, maintaining proper storage conditions is crucial to ensure these medications remain effective.
Why Temperature Control Matters for GLP-1 Medications
GLP-1 medications are peptide-based biologics, meaning they are made up of delicate peptide chains that need to stay intact to function properly. Unlike traditional chemical tablets that can withstand room temperature, these biologics are highly sensitive to heat, freezing, and sudden temperature changes. Such conditions can cause the peptides to lose their structure, clump together, or degrade, ultimately reducing their effectiveness. Even slight deviations from the recommended storage range of 2–8 °C can compromise the medication's quality.
In Singapore's hot and humid environment, prolonged exposure to high temperatures, freezing, or even leaving the medication in a warm car can render it ineffective. This underscores the importance of strict cold chain management during manufacturing, storage, and delivery. Ensuring that every pen reaches your fridge at the right temperature is critical to preserving its potency and safety.
Main Elements of Cold Chain Logistics for GLP-1 Medications
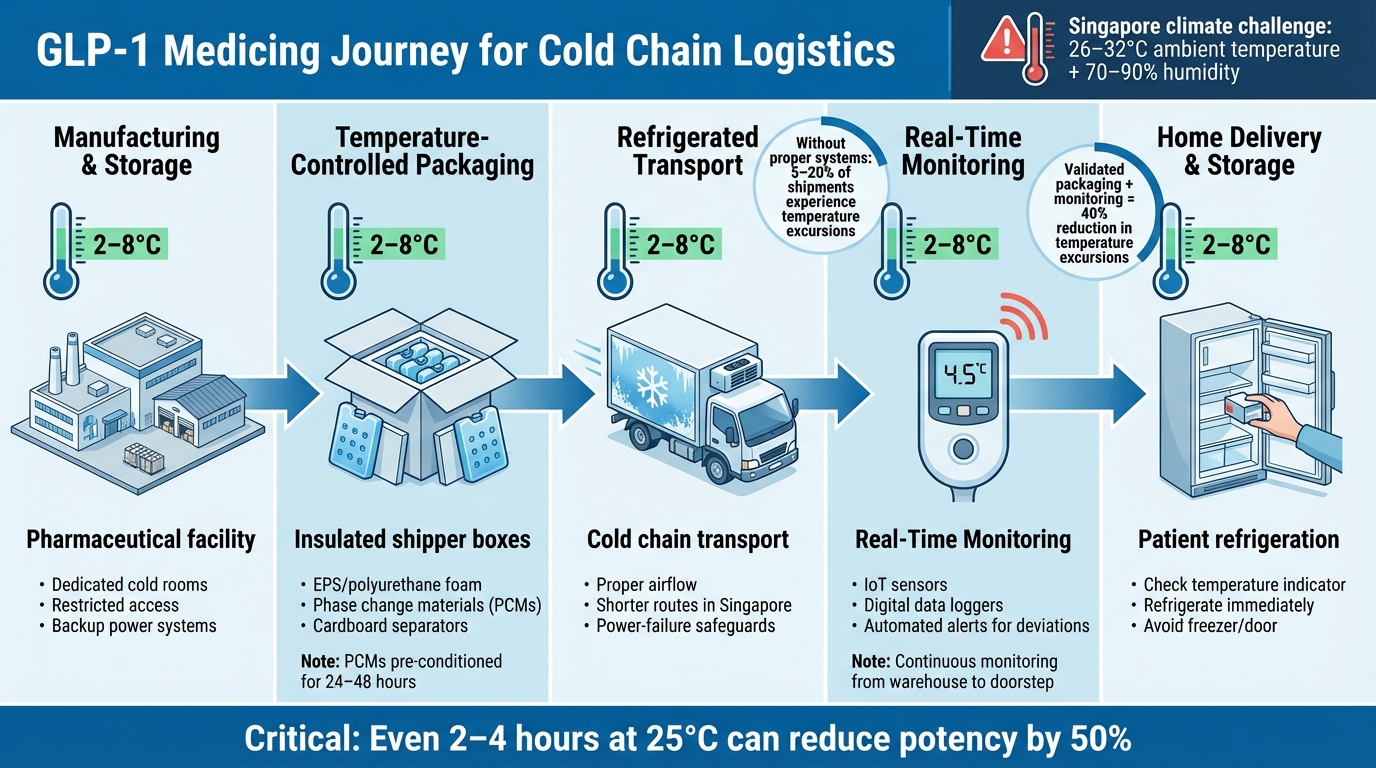
Cold Chain Journey for GLP-1 Medications: From Manufacturing to Your Fridge
Ensuring GLP-1 medications stay within the recommended temperature range, from production to your fridge, requires a seamless integration of three critical systems. Each plays a vital role in safeguarding these temperature-sensitive treatments, particularly in Singapore's consistently hot and humid climate, where temperatures often exceed 30 °C.
Temperature-Controlled Packaging
The first line of defence in protecting GLP-1 medications is insulated shipper boxes. These are typically crafted from materials like pharmaceutical-grade EPS, polyurethane foam, or vacuum-insulated panels, which act as a shield against external heat. Inside these boxes, phase change materials (PCMs) or gel packs, specifically designed to maintain a stable temperature of 2–8 °C, ensure the contents remain cool for 72–96 hours - even during extended transit times.
To work effectively, PCMs need to be pre-conditioned in a controlled refrigerator for 24–48 hours to reach the correct starting temperature. Over-frozen packs can freeze the medication, while under-conditioned ones won’t provide sufficient cooling. Cardboard separators are often used to prevent direct contact between refrigerants and medication, avoiding cold spots that could damage the product.
Given Singapore's tropical climate, delivery providers use extended-duration packaging systems, specifically tested for such conditions. These packages are clearly labelled for easy identification, allowing you to transfer your medication into your fridge immediately upon arrival. This packaging system forms the foundation for secure refrigerated transport.
Refrigerated Transport Systems
Once securely packaged, GLP-1 medications rely on refrigerated transport systems to maintain temperature stability during bulk distribution. Vehicles like refrigerated vans and trucks are calibrated to keep cargo areas between 2–8 °C, ensuring safe transport between warehouses, pharmacies, and distribution centres. These vehicles are designed with proper airflow systems to prevent parcels from coming into contact with freezing evaporator outlets or warmer areas near doors.
In Singapore’s urban environment, shorter and more frequent delivery routes reduce the time doors are open, minimising temperature fluctuations. Facilities handling these medications are equipped with dedicated pharmaceutical refrigerators or cold rooms. These storage units are continuously monitored, have restricted access, and follow strict protocols for receiving, storing, and packing stock. Backup systems, such as power-failure safeguards, ensure protection against unexpected disruptions like traffic delays or equipment malfunctions.
Temperature Monitoring Equipment
Temperature monitoring is the glue that holds the cold chain together, bridging both packaging and transport to ensure the integrity of GLP-1 medications. Tools like digital data loggers, IoT-enabled sensors, and chemical indicators track temperature conditions at every stage of the process. Fixed sensors in warehouses and on transport vehicles provide real-time data, with automated alerts triggered by any deviations.
Individual parcels often include single-use data loggers or excursion indicators placed alongside the medication. These devices capture the actual product-level temperature throughout the journey. If temperatures approach unsafe levels, alerts can prompt immediate actions, such as rerouting, prioritising delivery, or even recalling the parcel before it reaches your home. Archived temperature data also supports quality assurance, regulatory compliance, and investigations, ensuring every dose of GLP-1 medication remains effective and safe from the pharmacy to your doorstep.
Challenges in Maintaining Cold Chain for GLP-1 Medications
Even with advanced packaging and transport systems, keeping GLP-1 medications within the required temperature range remains a tough task. These medications are highly sensitive, and even brief temperature fluctuations can compromise their effectiveness. With global prescriptions skyrocketing by about 300%, the strain on logistics networks has increased, raising the risk of disruptions.
Common Cold Chain Problems
The most common issue in the cold chain is temperature excursions - when medications fall outside the critical 2–8 °C range, even for a short time. Without proper backup systems, 5–20% of shipments encounter such excursions. Research shows that short exposures (e.g., 2–4 hours at 25 °C) can cut a drug's potency by half, while longer deviations can render entire batches unusable.
Even with insulated packaging and digital monitoring, several factors can disrupt the cold chain. One major issue is inconsistent refrigerant conditioning. Phase change materials that aren’t pre-cooled for 24–48 hours often fail to regulate temperatures effectively. Delays during loading, customs clearance, or last-mile delivery can also push transit times beyond packaging limits. For instance, an 8-hour delay in customs caused GLP-1 shipments to reach 15 °C, leading to 25% of the batch being discarded.
Packaging failures are another concern. Damaged insulated shippers, punctured gel packs, and rough handling expose medications to external heat. Studies confirm that poor packaging significantly increases failure rates. Additionally, warehouse or vehicle power outages can cause rapid temperature spikes, and gaps in real-time monitoring mean some excursions go unnoticed until it’s too late.
Managing Cold Chain in Singapore's Hot Climate
Singapore’s tropical climate adds another layer of complexity. With temperatures ranging from 26–32 °C and humidity levels between 70–90%, heat penetrates packaging more quickly, shortening the hold time for refrigerated shipments. Solutions designed for cooler climates often fall short here, especially during last-mile delivery when parcels may sit in non-air-conditioned vehicles or at doorsteps.
To tackle these challenges, packaging systems validated for high heat and pre-conditioned refrigerants that resist rapid thawing are essential. Afternoon rain delays further extend exposure times, stressing the cold chain. Experts suggest several strategies to mitigate these risks:
- Route optimisation to reduce outdoor exposure times.
- Seasonal pack-out designs tailored to Singapore’s conditions.
- IoT sensors that provide real-time alerts for temperature and humidity changes.
Such measures have shown promising results. For example, validated packaging combined with automated monitoring has cut temperature excursions by 40% in Singapore’s challenging environment.
sbb-itb-5db499f
How Telehealth Clinics in Singapore Handle Cold Chain Logistics
Telehealth clinics in Singapore that prescribe GLP‑1 medications face the added challenge of ensuring these drugs are safely delivered to patients' homes. To meet this challenge, these clinics must strictly follow the regulations set by the Ministry of Health (MOH) and the Health Sciences Authority (HSA), ensuring that the medications remain effective and safe throughout the delivery process.
Meeting Singapore's Healthcare Regulations
Telehealth clinics are classified as healthcare institutions under the Healthcare Services Act (HCSA). This means they must meet MOH licensing requirements, which include having robust systems in place to manage the storage, handling, and delivery of medications. Additionally, the HSA’s Good Distribution Practice (GDP) and Good Storage Practice (GSP) guidelines require that temperature-sensitive medications, such as GLP‑1 drugs, are stored and transported within a strict temperature range of 2–8°C. This involves using calibrated equipment and maintaining detailed temperature logs.
Maintaining consistent temperature control is crucial from the clinic to the patient’s home. Any deviations in temperature must be quickly investigated, documented, and corrected. Clinics approved by the MOH, such as Trimly, work closely with GDP-compliant pharmacies and logistics providers. These partnerships are formalised through written agreements that clearly outline the responsibilities of each party in the cold chain process.
Home Delivery with Temperature Control
To ensure the integrity of medications during last-mile delivery, telehealth providers use validated insulated packaging with phase change materials (PCMs) and real-time IoT monitoring systems. These measures ensure that the 2–8°C temperature range is maintained until the medication reaches the patient.
For instance, Trimly uses temperature-controlled couriers equipped with integrated monitoring systems to deliver GLP‑1 medications. The packages come with clear instructions for immediate refrigeration, and free follow-ups are offered to verify delivery and replace medications if necessary. By carefully planning delivery routes and schedules, providers ensure that medications remain safe and effective until they are stored in the patient’s refrigerator.
How to Check and Store Your GLP-1 Medication at Home
When your GLP-1 medication arrives at your doorstep, the responsibility of maintaining its cold chain falls on you. Proper handling and storage at home are crucial to ensure the medication remains safe and effective throughout your treatment.
What to Check When Your Medication Arrives
As soon as your package is delivered, open it without delay. Singapore's warm climate can quickly expose the medication to unsafe temperatures if left unpacked for too long.
Start by checking the temperature indicator or data logger included in the package. Many GLP-1 shipments come with a single-use indicator card that changes colour if the temperature has gone outside the safe range of 2–8°C. If the indicator shows a "fail" or "excursion" symbol, do not use the medication. Instead, contact your pharmacy or telehealth provider immediately to arrange for a replacement.
Next, inspect the packaging. The insulated container should still feel cool, and the gel packs inside should be at least partially frozen or cold to the touch. If the gel packs are completely warm or the box feels hot, this could indicate a loss of temperature control. Look for any signs of damage, such as leaks or crushed areas.
Finally, examine the medication itself. The GLP-1 pen or vial should contain a clear, colourless solution. If you notice discolouration, cloudiness, particles, or clumps, do not use it. Check the pen or vial for damage or leaks as well. If you see frost, ice crystals, or condensation inside, the medication may have been frozen by mistake - consult your healthcare provider before use.
Also, double-check the label to ensure it includes the correct medication name, strength, patient details, and expiry date.
Once everything checks out, move the medication to its proper storage location immediately.
Storing GLP-1 Medications in Your Refrigerator
Place your medication in the refrigerator without delay. It should be stored at 2–8°C, ideally in the main compartment. Avoid the door and freezer sections, as these areas are prone to temperature fluctuations.
Keep the pen or vial in its original carton on a middle shelf, away from the back wall and cooling vents. This helps maintain a stable temperature. To ensure accuracy, consider placing a fridge thermometer on the same shelf. Keep the medication separate from raw foods to maintain hygiene.
Avoid common mistakes like stacking medications against freezer plates or leaving them at room temperature for too long. In Singapore's humid and hot environment, even brief exposure to temperatures above 30°C can degrade the medication. If the medication has been left in a car or exposed to extreme heat, contact your pharmacist or healthcare provider before using it.
Some GLP-1 pens, after their first use, can be stored at room temperature (usually below 25–30°C) for a limited time. Always refer to the instructions in your medication guide. To keep track, mark the date of first use on the carton and dispose of the pen once its in-use period has passed, as its stability and sterility cannot be guaranteed beyond that point.
Telehealth services like Trimly offer additional support, including detailed checklists, video tutorials, and free follow-up consultations. These resources can help you troubleshoot refrigerator issues, interpret temperature indicators, and plan safe storage - whether at home, during travel, or when your fridge is heavily used. This helps minimise waste and ensures your medication remains effective throughout your treatment.
Conclusion
Ensuring proper cold chain logistics is essential for the safe and effective use of GLP‑1 medications. These temperature-sensitive drugs must consistently remain between 2–8°C, from the manufacturing stage to storage at home. Even a short exposure to extreme heat or freezing temperatures can compromise their effectiveness.
Every step in the supply chain plays a vital role. From insulated packaging with specialised materials to refrigerated transport and real-time temperature monitoring, each measure helps protect these medications from temperature fluctuations. Clear instructions for patients further reinforce this chain. In Singapore's hot and humid weather, strict compliance with regulatory standards becomes even more important. Telehealth clinics like Trimly address these challenges by delivering GLP‑1 medications in temperature-controlled packages equipped with real-time monitoring.
Patients also play a crucial role in preserving the cold chain. Upon receiving the medication, it’s important to check the temperature indicators, refrigerate the product immediately, and promptly contact a healthcare provider if any issues with temperature control are noticed.
FAQs
How can I make sure my GLP-1 medication stays effective during delivery?
To ensure your GLP-1 medication remains effective, it’s important to store it at the proper temperature - between 2°C and 8°C. Keep it away from extreme heat and avoid freezing it, as these conditions can compromise its quality.
When your medication arrives, check that it’s packed in insulated materials to maintain the required temperature during transit. Once received, transfer it to the refrigerator right away to preserve its effectiveness.
What should I do if the temperature indicator shows an issue when I receive my medication?
If the temperature indicator signals a problem when your medication arrives, contact Trimly right away through their online chat or support channels. Share all relevant details, including any noticeable signs of temperature-related issues.
Do not use the medication until a healthcare professional verifies it is safe. Trimly will provide guidance on what to do next, ensuring your treatment stays both effective and safe.
Why should GLP-1 medications be stored in the main section of the fridge?
Storing GLP-1 medications in the main compartment of the fridge is essential for maintaining their stability and effectiveness. Unlike the fridge door or other sections, which are prone to temperature fluctuations from frequent opening and closing, the main compartment provides a more consistent environment.
By keeping these medications in the main section, you help ensure they stay within the recommended temperature range, preserving their quality and ensuring they perform as expected. Always refer to the storage guidelines that come with your medication to ensure proper handling.
.png)

